Background
The presence of stable plateaus in Kaplan-Meier (KM) survival analyses of clinical trials with ≥5 years of median follow-up raises the issue of the curative potential of immunotherapies for patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL). Mixture cure models (MCM), unlike standard survival analyses, stratify study populations into long-term survivors (LTS) and non-LTS based on statistical modeling approaches. LTS are assumed to not experience a disease-related event (termed “cured” in only a statistical sense) and are assumed to have an all-cause mortality of the age- and gender-matched general populace. Non-LTS experience events based on the outcomes of the underlying study. Based on these analyses, life expectancies for the combined (LTS and non-LTS) study population are estimable (Felizzi F et al. 2021).
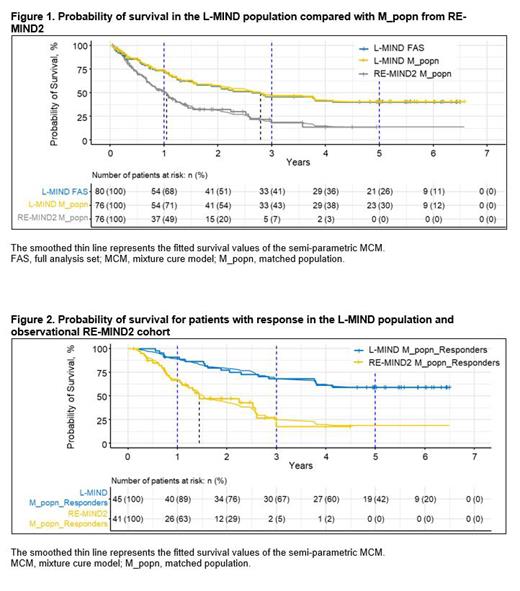
The final 5-year results of the Phase II L-MIND study (NCT02399085; Duell J et al. 2023) of tafasitamab + lenalidomide (LEN) in patients with R/R DLBCL identified an LTS population, making the study data suitable for MCM analysis. For contextualization, patients from the retrospective, observational RE-MIND2 study (NCT04697160; Nowakowski G et al. 2022; Nowakowski G et al. 2023) who were treated with a wide variety of systemic therapies including polatuzumab vedotin / bendamustine / rituximab (pola-BR) were matched to the L-MIND population. We used various MCM analyses to estimate the proportion of LTS and overall life expectancy in patients from L-MIND compared with matched RE-MIND2 cohorts.
Methods
Patients from the full analysis set (FAS) from L-MIND (n=80) were 1:1 matched without replacement with a RE-MIND2 comparator cohort, based on 9 covariates originally defined in the RE-MIND2 study, generating 76 matched pairs (M_popn). Two subsets of patients from L-MIND were also defined: those with only one prior line of therapy (pLoT; 1:1 matched to a corresponding RE-MIND2 cohort) generating 39 matched pairs; and those experiencing a response to therapy (non-matched subsets from the 1:1 matched populations). KM curves for the comparisons are displayed, and a semi-parametric MCM was used to estimate the proportion of LTS and combined life expectancy (accounting for LTS and non-LTS) within each population (Cai C et al. 2021; Gressani O et al. 2022). The life expectancy of the general population was estimated using data from the US National Vital Statistics System. Additional MCMs were generated for sensitivity analyses.
Results
KM curves show improved survival outcomes in the L-MIND M_popn treated with tafasitamab + LEN with up to 5 years of follow-up compared with RE-MIND2 M_popn receiving standard-of-care systemic therapies in an observational setting( Figure 1). The LTS proportion of the L-MIND M_popn was estimated as 40%, and the combined life expectancy was 7.27 years (95% confidence interval [CI]: 5.56-8.93 years). By comparison, the RE-MIND2 M_popn had a LTS proportion and a combined life expectancy of 13% and 2.93 years (CI: 1.68-4.86 years), respectively. The probability of survival among patients with response to therapy in L-MIND or in the observational RE-MIND2 setting is shown in Figure 2. In patients with an objective response in L-MIND M_popn, the LTS proportion was estimated as 59%, with a combined life expectancy of 9.97 years (CI: 7.69-11.79 years). For the subset of patients with response in RE-MIND2 M_popn, the LTS proportion and combined life expectancy were 18% and 3.73 years (CI: 1.99-6.51 years), respectively. Half of the patients in L-MIND received tafasitamab + LEN as second-line therapy; the LTS proportion in this subset was 51%, with a combined life expectancy of 8.24 years (CI: 5.96-10.21 years). Additional subgroup and sensitivity analyses will be presented later.
Conclusions
The MCM analysis of 5-year data from the L-MIND study suggests that treatment with tafasitamab + LEN tripled the proportion of LTS (40%) when compared with matched real-world patient cohorts receiving systemic therapies (13% LTS). Among patients responding to treatment, the proportion of LTS increased to 59% for those receiving tafasitamab + LEN, compared with 18% for other systemic treatment. The increases in LTS proportion resulted in substantial increases in estimates of life expectancy; these exploratory data warrant further examination in larger patient cohorts.
Disclosures
Duell:MorphoSys AG, Regeneron: Research Funding. Dreyling:Abbvie, Astra Zeneca, Beigene, BMS/Celgene, Gilead/Kite, Janssen, Lilly/Loxo, Novartis, Roche: Other: Scientific advisory boards; Astra Zeneca, Beigene, Gilead/Kite, Janssen, Lilly, Novartis, Roche: Honoraria; Abbvie, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Roche: Research Funding. Gaidano:Abbvie and Janssen: Speakers Bureau; Abbvie, Astra-Zeneca, BeiGene, Incyte, Janssen, Lilly: Other: Advisory board. Kalakonda:BMS/Celgene, Gilead/Kite, Hospira, Incyte, Janssen, Karyopharm, Roche, Takeda, AbbVie: Other: Advisory board, Speakers Bureau; Celgene, Gilead, Roche: Research Funding. Gonzalez Barca:Janssen, Abbvie, AstraZeneca: Other: Travel; Janssen, Abbvie, Takeda, EUSAPharma, AstraZeneca, Lilly: Speakers Bureau; Janssen, Abbvie, Kiowa, EUSA Pharma, Beigene, Sobi: Consultancy. Liberati:Novartis, Janssen, AbbVie, Roche, Amgen, Celgene, Bristol-Myers Squibb, Incyte, Pfizer, Doxopharma, Verastem, BeiGene, Oncopeptides, Karyopharm, Archigen, CTI BioPharma, Debiopharm, MorphoSys AG, FibroGen, MEI Pharma, Regeneron, Dr Reddy's Laboratories Sp: Research Funding; Bristol-Myers Squibb, Servier, Celgene, AbbVie, Amgen: Honoraria; Abbvie, Amgen, Takeda, Servier: Membership on an entity's Board of Directors or advisory committees; Incyte, Event srl: Consultancy. Nagy:Takeda, Janssen, Abbvie, Roche, Amgen, Servier, Astellas: Consultancy. Moik:MorphoSys AG: Current Employment, Current equity holder in publicly-traded company. Vukcevic:MorphoSys AG: Current Employment. Amin:MorphoSys AG: Current Employment; Paion AG: Current equity holder in publicly-traded company. Bakuli:MorphoSys AG: Current Employment; Ludwig-Maximilians-University Hospital, Munich, Germany: Consultancy.